In the emergency department, the patient is awake but tired and has resting dyspnea. Cheyne-Stokes breathing pattern is noted (frequency of 30 respirations/minute with few seconds of apnea intermittently). She has central and peripheral cyanosis and is peripherally cold, her skin is not marmorized or clammy. Vital signs show varying blood pressure, initial measurement 128/70, pulse 90/min. Intermittently her systolic pressure is as low as 75mmHg. Pulsoximeter shows show saturation of 74% with 3L O2, it has been placed on both hands and even earlobes and always has same values. She is afebrile.
Patient has a previous history of 3-vessel coronary disease and end-stage heart failure, EF has previously been evaluated as 15-20%.
Patient was evaluated as critically ill but did not show clincal signs of immediate threatening circulation or organ failure; she was awake and alert so we decided we had some time to work her up and wanted to start with blood samples, first of all blood gas. The patient was cachetic and we had a hard time finding proper, pulsating arteries; the radial pulses could not be found and the inguinal ones were very vague.
 The risks involved in punctuating an artery are infection, bleeding and hitting other structures such as the accompanying femoralis nerve.
The risks involved in punctuating an artery are infection, bleeding and hitting other structures such as the accompanying femoralis nerve.Using sterile techniques the infection risk can be minimized and the fact that we are only punctuating, not inserting a catheter, makes the risk even lower. Bleeding risk with a small needle such as the one mounted to the blood gas syringe is minimal. The risk of hitting the femoral nerve is overestimated, especially if ultrasound is used where the needle can be seen to hit the artery and nothing else. Even in the case of touching or even penetrating the thick sheath of the femoralis nerve, the risk of permanent damage is astronomical with needle so small. This has been thoroughly documented in the literature from research of femoral nerve blocks where complications are extremely rare. Expect the femoral artery at 1,5cm depth in the normal-sized patient and expect problems in obese patients where it may lay as deep as 5-7cm, far beyond reach for the short ABG needle.
Despite very low risk of injury, punctuating the femoralis artery (or vein even) is in my opinion rarely seen unless in extremis such as cardiac arrest and should be considered as a valid option when other sites are not possible.

An alternative for those not so intrigued would be to find the brachialis artery which in most patients is easily palpated in the antecubital fossa, between the medial epicondyle and biceps brachii tendon.
The brachialis artery lies much deeper and will commonly move away from needle and thus harder to get to. It is though though commonly used in pediatrics where it is easier to maneuver.
A decent brachialis vein was seen and a venous blood gas (VBG) was drawn, revealing the following values:
- pH 7.280
- pCO2 6.42 kPa
- pO2 2.74 kPa
- Na 128
- K 4.8
- Crea 86 umol/L
- Ca 1.13 mmol/L
- Cl 95 mmol/L
- Glu 6.1 mmol/L
- Lactate 7.0
- Hb 153 g/L
- CO-Hb 6.9%
- MetHb 0.8%
- calcluated SatO2 27.6%
- HCO3 19.0mmol/L
- BE -3.8mmol/L
A lactate of 1.0 was found only a week ago. This VBG shows a state of mixed respiratory (uncompensated) and metabolic acidosis with normal anion gap (14) - most likely explained by lactic acidosis. As expected, the patient is sick! Increased lactate tells us that tissues are not being perfused adequately and most likely this is because of the heart failure and impending respiratory failure - the patient was getting tired of prolonged hyperventilation and needs help.
A VBG has been show to correlate very well with ABG except for very high pCO2 states, uncommonly encountered and mostly irrelevant (have you heard of the patient who was incredibly hypercapnic? Would you run faster than if he was "just" hypercapnic?). For obvious reasons, VBG cannot measure PO2 since it is always presumed to be arterial and more commonly denoted as PaO2 (note that extra "a") to indicate it's arterial origin. Thus the calculated SaO2 value will always be wrong from a VBG - something I learned by error in this case!
But more important is the distinction between PaO2 and SaO2, mistakenly believed to correlate pretty well. After all they both measure the amount of oxygen in the blood. But PaO2 does not measure effective oxygen, ready for use by the tissues. It's just free O2 molecules and they need to be bound to hemoglobin to be of any use peripherally in tissues. Indeed, too high PaO2 (eg FiO2 100% for longer periods) sets ground for harmful free radicals - Amal Mattu recently had a great post on this on EmRap, reminding us to use O2 sparingly in the post-resuscitative phase after cardiac arrest.
Nontheless - PaO2 clearly indicates how much O2 the patient is taking in through the alveoli and low values suggest you should increase FiO2 and/or assist ventilation and even intubate if everything else fails. What PaO2 does not indicate is if the tissues are *receiving* O2 - the intubated patient on 100% FiO2 can die from hypoxia if hemoglobin is not working (eg. CO poisoning, severe anemia) or perfusion decreased (heart failure, severe bleeding). Indeed, that's the definition of shock, whatever it's cause.
The "perfusing O2" in blood is called 'oxygen content', CaO2 and it's value is calculated by the following formula:
CaO2 = SaO2 * 1.34 * Hb + 0.003 * PaO2
Which underscores the above; perfusing oxygen is literally independent on PaO2. To actually measure tissue perfusion (or hyperperfusion to be accurate), we need... lactate (there are more advanced tools in the ICU eg. Picco)! It's not the most important clinical knowledge but one of the cornerstones of understanding O2 in clinical medicine and reminder to the physician to not only look at the PaO2 value but the whole clinical picture. Excellent in-depth explanation of difference between pO2 and SatO2 Amal Mattu on EmRap: Post Cardiac Arrest Syndrome
Because of hypotension the nurse was getting impatient and wanted to start fluids. A more thorough examination is done:
- Skin: no turgor but general, diffuse pitting edema of whole body, hands, feet, sacrum and even up to flanks.
- Cardiac: distant heart sounds, possibly S3 and a pansystolic murmur. Neck vein distension.
- Lungs: normal respiratory sounds, no crackles, no wheezing heard.
- Bedside ultrasound: a large liver but IVC was hard to visualize properly (this was in my first months of doing ultrasound, no pleural windows were done!). A rough "ECHO" shows all four chambers diffusely dilated and severe global hypokinesia of left ventricle, EF estimated 5-10%.
A chest x-ray is done revealing considerable amounts of pleural fluid on right side, none on left. Slightly dilated central veins, no edema, no infiltrates.
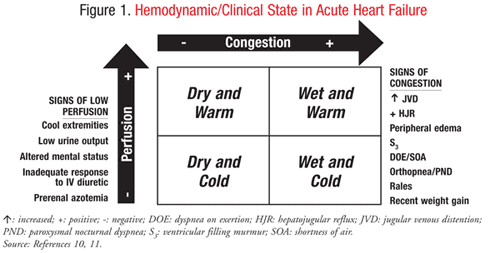 This is a topic of great debate and a very interesting one as myths have been debunked and treatment protocols changed in only recent years (eg. Morphine is now out of AHF treatment unless palliative). All in all - recent studies have taught us that we have been doing it wrong for a long time and there is no single approach to the acute heart failure patient. The Forrester classification is an excellent categorization into four general categories of warm/cold (degree of perfusion where cold is hypoperfused as in this case) and wet/dry (with regard to pulmonary edema - our patient is dry). The different types of AHF need different treatment modalities and the patient above is a cold/dry one (with regards to pulmonary edema - chronic heart failure has collected edema peripherally with pitting edema, neck vein- and liver stasis).
This is a topic of great debate and a very interesting one as myths have been debunked and treatment protocols changed in only recent years (eg. Morphine is now out of AHF treatment unless palliative). All in all - recent studies have taught us that we have been doing it wrong for a long time and there is no single approach to the acute heart failure patient. The Forrester classification is an excellent categorization into four general categories of warm/cold (degree of perfusion where cold is hypoperfused as in this case) and wet/dry (with regard to pulmonary edema - our patient is dry). The different types of AHF need different treatment modalities and the patient above is a cold/dry one (with regards to pulmonary edema - chronic heart failure has collected edema peripherally with pitting edema, neck vein- and liver stasis). Diuretics may be causing more harm than good as admitted patients get electrolyte imbalances and kidney failure. Their immediate circulatory effects are minimal and very short living and thus doubtful if they fit in AHF treatment at all. Inotropic medicines such as dopamine or simdax are much more relevant in this scenario, even vasopressors to induce better perfusion to tissues.
As Amal Mattu has so excellently pointed out, what seems “most correct” is to use diuretics to treat volume overload, not AHF by itself. If the lungs are full of edema because the whole body is and that fluid puts even more strain on the decompensated heart - whole body fluid needs to be removed. But these patients most commonly will present with acute onset of symptoms and with high blood pressure and need nitro and CPAP, not diuretics. These are the SCAPE patients, standing for “Sympathetic Crashing Acute Pulmonary Edema”. Scott Weingart’s podcast about SCAPE is a must listen as you will encounter these patients often in the ED and with no time to prepare yourself. They will be terrified when you see them because their adrenaline levels are sky high - thus the hypertension. European Heart 2005 guidelines on acute heart failure
Cardiology was consulted and was a little puzzled on the diuretics question but decided it was worth trying small dose lasix on the assumption that some inotropy (contractility) might be gained by shifting the Frank-Sterling curve. Patient was not obviously dehydrated and hypotension is most likely because of low cardiac output. In the cardiology unit Simdax and vasopressors (noradrenaline) were infused to treat a previous diagnosed 'dilated cardiomyopathy' on ischemic basis. The patient had previously stated she didn't want to be operated and only wanted medicines for symptomatic relief.
Major learning points from case
- Standard pulsoximeters in the ED cannot be relied on for SaO2 in heart failure or any form of decreased circulation.
- VBG can be drawn from femoral vein, as long as sterile technique is used and navigated by ultrasound.
- VBG correlates excellently with ABG values except for PO2, in low perfusion states an ABG must be used since the pulsoximeter gives false values.
- Hypotension does not equal hypovolemia!
- Diuretics should not be pushed thoughtlessly in acute heart failure and are seldomly first line treatment anymore - not even in acute, pulmonary edema or congestive heart failure!



.jpg)


